DFE Pharma launches Continuous Manufacturing platform in collaboration with Gericke
Platform integrates CM-ready excipients, testing environment and process expertise
DFE Pharma has announced the launch of its Continuous Manufacturing (CM) platform to support pharmaceutical companies in formulation development, optimisation and lifecycle management of CM processes. The platform has been developed in collaboration with Gericke, a company specialising in feeding, blending and powder processing equipment. The initiative combines CM-ready excipients, a CM evaluation environment and multidisciplinary expertise to enable data-driven decisions from early exploration through optimisation and long-term CM operations.
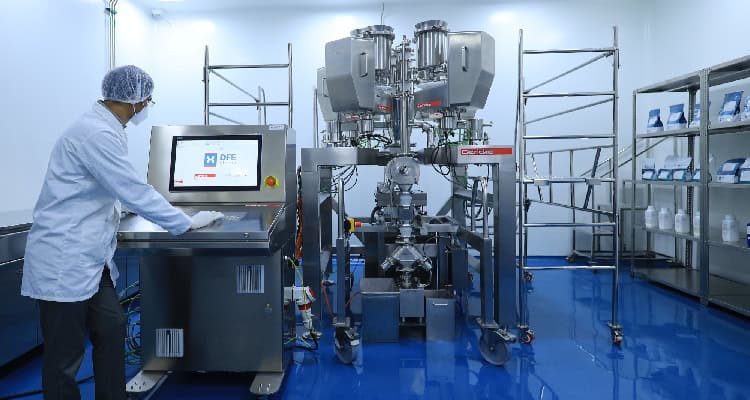
As part of the platform, DFE Pharma has established continuous manufacturing capability at its C2F Center of Excellence in Hyderabad, India. The facility features a Gericke Formulation Skid (GFS) with modular feeding and blending options. The skid is installed in a non-GMP environment to allow process assessment and optimisation without affecting existing GMP manufacturing or requiring upfront infrastructure investment. The modular configuration at the C2F Center integrates the GFS with pre-blending, downstream processing and analytical capabilities to generate data for decision-making. The equipment allows pharmaceutical companies to observe continuous feeding and blending operations, test formulation strategies using CM-ready materials and evaluate how data supports implementation.
DFE Pharma provides a portfolio of excipients characterised for performance under continuous processing conditions. These excipients are intended to support processing, system design and lifecycle performance, enabling manufacturers to manage material variability and meet regulatory requirements during development and commercialisation.
The platform is supported by DFE Pharma’s expertise in formulation development, analytics, process understanding and variability science, along with Gericke’s expertise in continuous processing equipment. The collaboration is designed to assist pharmaceutical companies in implementing development and registration strategies aligned with regulatory principles, including ICH Q13.
Dr Sven Abend, Chief Executive Officer at DFE Pharma, said, “Continuous Manufacturing is reshaping how medicines are developed and produced, but success depends on making the right choices at every stage. With our Continuous Manufacturing platform, we are bringing together our CM-ready excipients, a flexible testing environment, and applied scientific expertise. We are sure this platform will enable our customers to make informed, data-driven CM decisions from early exploration through optimisation and long-term operation with confidence.”
Markus Gericke, CEO at Gericke Group, said, “This platform brings together our applied expertise in formulation development, powder handling, and continuous processing science. At Gericke, we contribute decades of engineering and process knowledge, helping customers interpret CM data, understand material–equipment interactions, optimise key parameters, and align their strategies with guidelines such as ICH Q13. Together, we provide clear, science-based direction that supports a smooth transition to robust Continuous Manufacturing.”
Continuous manufacturing is a process in which raw materials are fed, transformed through controlled unit operations and discharged as finished product. It supports product quality, process control and equipment use compared to batch manufacturing. Pharmaceutical companies have integrated continuous processes into parts of their operations, while others are progressing towards CM. Adoption can involve investment requirements, regulatory and operational considerations and the need for process understanding.