What It Takes to Build Pharmaceutical Facilities That Perform from Day One
As regulatory expectations tighten and speed-to-market pressures intensify, pharmaceutical facilities must be designed for certainty from day one. Drawing from large-scale project experience, Mr Subhendu Mohanty, Vice President, Projects - Pharma Access outlines how integrated engineering, simulation-led design, and early validation are redefining successful facility delivery
The pharmaceutical facility landscape has become increasingly complex. Regulatory expectations, operational efficiency, sustainability requirements, and speedto-market pressures must all be addressed simultaneously. From my experience, the single biggest pitfall companies face when starting a major construction or expansion project is the lack of upfront integration.
Building a modern pharmaceutical facility is not just about civil construction. It requires the seamless integration of process design, utilities, automation, and regulatory compliance, including USFDA guidelines, cGMP, Annex 1, and other global regulatory frameworks. As I often say, “One of the biggest challenges in pharma projects is anticipating compliance and operational needs before the first brick is laid.” When integration is considered late in the process rather than from the very start, it leads to fragmented execution and costly rework.
Integration from Conceptualisation to Commissioning
At Pharma Access, we fundamentally change the traditional approach to designing and building pharmaceutical facilities through what we call Integration from the Ground Up.
We embed compliance and efficiency from the outset. By incorporating regulatory considerations into every phase of the project, from conceptualisation to commissioning, we ensure the final facility is not only compliant but also optimised for seamless, high-performance operation. This integrated approach reduces execution risk and enables smoother commissioning and validation.
Simulation-Based Engineering and Digital Twins
Our team relies heavily on Simulation-Based Engineering, because simulation is a powerful predictive tool.
Simulation allows us to foresee potential issues and optimise systems in ways that are simply impossible with conventional drawings. We build complete digital twins of pharmaceutical facilities, including process flows, HVAC systems, piping, and electrical circuits. These virtual replicas allow us to anticipate operational challenges, optimise layouts, and drastically reduce the risk of costly field modifications.
A key application of this approach is cleanroom design. We use Computational Fluid Dynamics (CFD) simulations to guarantee adherence to ISO 14644 standards. By calculating the precise Air Changes Per Hour (ACPH), where airflow rate and room volume are balanced, we ensure proper contamination control while maintaining energy efficiency. This is a critical metric for aseptic manufacturing environments.
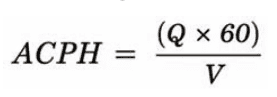
Here, Q is the airflow rate (ft³/min) and V is the room volume (ft³).
Quality by Design as a Planning Principle
Quality by Design means quality is engineered in, not inspected in. By embedding Quality by Design (QbD) into the initial planning stages, we are able to anticipate process variability and embed controls proactively. This allows us to identify Critical Quality Attributes (CQA), Critical Material Attributes (CMA), and Critical Process Parameters (CPP) early in the project lifecycle.
This synergistic design, where digital twin insights inform optimisation and QbD principles embed controls, guarantees consistent, high-quality outcomes across the facility lifecycle.
De-Risking Timelines Through FEL and Early CQV
Project delays and compliance issues are costly in pharmaceutical manufacturing. To de-risk timelines and budgets, we rely on two cornerstone philosophies, Front-End Loading (FEL) and early integration of CQV.
Front-End Loading is intensive upfront planning to define scope, technical requirements including URS, costs, and schedules before significant capital commitment. This approach minimises scope creep and reduces downstream uncertainty.
At the same time, embedding CQV early is a game-changer. It ensures all systems are designed for validation from day one. We develop a comprehensive Validation Master Plan detailing URS, FAT, SAT, IQ, OQ, and PQ. This ensures documentation is audit-ready for USFDA inspections and compliant with regulations such as 21 CFR Part 11, preventing costly surprises and enabling immediate operational readiness after handover.
Sustainability and Safety as Design Pillars
Sustainability and safety are not afterthoughts. They are woven into the design DNA of every project we deliver.
Classified cleanrooms are known for over-ventilation. By using validated CFD modelling, we precisely optimise airflow and typically achieve a 10 to 25 percent reduction in energy use on HVAC systems alone. In parallel, we integrate Zero Liquid Discharge (ZLD) systems into our designs, preventing 70 to 80 percent of wastewater from being lost to drainage. This is crucial for achieving water neutrality and long-term sustainability.
We track this performance through our Sustainability Index, which is based on energy savings, water conservation, and material optimisation.
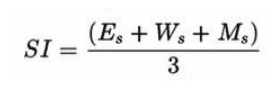
Turnkey Delivery and Single-Point Responsibility
Our approach provides seamless integration from conceptual engineering through procurement, construction, commissioning, and validation. As our director, Mr. Shams Parvaz often says, “Our turnkey approach is not about speed alone. It is about delivering facilities that are ready to operate efficiently, safely, and compliantly from day one.” Clients benefit from a single point of responsibility, integrated project management, scalable cleanroom solutions, and a regulatory-aligned CQV strategy, eliminating friction between multiple stakeholders.
Modular Facilities and Future Agility
Modular and mobile facilities are gaining prominence as the industry demands speed-to market and flexibility. Modular construction allows activities to happen in parallel, with prefabricated modules built in controlled factory environments while site work progresses simultaneously.
This approach reduces timelines by 30 to 40 percent, improves quality and safety, provides scalability, and is inherently sustainable by design.
Engineering and Execution as One Philosophy
We often use the analogy of E=mc2 to describe our operational philosophy. It represents the fusion of engineering mastery with execution capability.
This philosophy captures how we combine advanced engineering, disciplined execution, Quality by Design, simulationled planning, and sustainability strategies to solve complex pharmaceutical facility challenges. The outcome is facilities that are operationally robust, compliant, and strategically transformative.
In today’s pharmaceutical environment, this integrated approach is no longer optional. It is essential.



