Generic drug development: The way forward
Anantha Keerthi, Partner, Vector Consulting Group, points out that pharma companies need to either launch many new speciality generic products (better margins) or hire extra resources to develop more regular generic drugs to counter eroding profitability
Even though the global generic drug market is growing at a CAGR of ~8 per cent, many Indian pharma companies are witnessing their profitability coming down continuously. This is one of the reasons for the recent devaluation of several pharma stocks in India. What is the reason behind this lull as far as the Indian life science companies are concerned? The pundits are unanimous in their response – changes in the US generic drug market.
The US is the biggest market for generic medicines (~90 per cent of prescriptions are fulfilled by generic drugs). The enactment of the 2012 Generic Drug User Fee Act (GDUFA) resulted in an increase in the Abbreviated New Drug Application (ANDA) approval rate from the US Food and Drug Administration (FDA) for Indian generics. But one of the consequences of this has also been an increase in competition and steady price erosion in the portfolio of drugs made by different life science companies.

What to do?
The options to counter this eroding profitability for these pharma companies are to either launch many new speciality generic products (better margins) or hire extra resources to develop more regular generic drugs. Neither of these is easy. Development of speciality drugs is complicated and time/ effort consuming (evidence – there are plenty of patent-expired branded drugs for which generics are yet to be developed).
The high attrition rate, difficulty in scaling up critical resources, etc. make the second option – recruitment of skilled resources – not an easy task either. The scarcity of experienced resources is evident in all life science companies. These resources are fully stretched out either trying to ensure that projects reach the Exhibit Batch (EB) stage in time (second quarter), struggling to get needed submission documentation in place (fourth quarter) or trying to respond fast to FDA queries (post submissions).
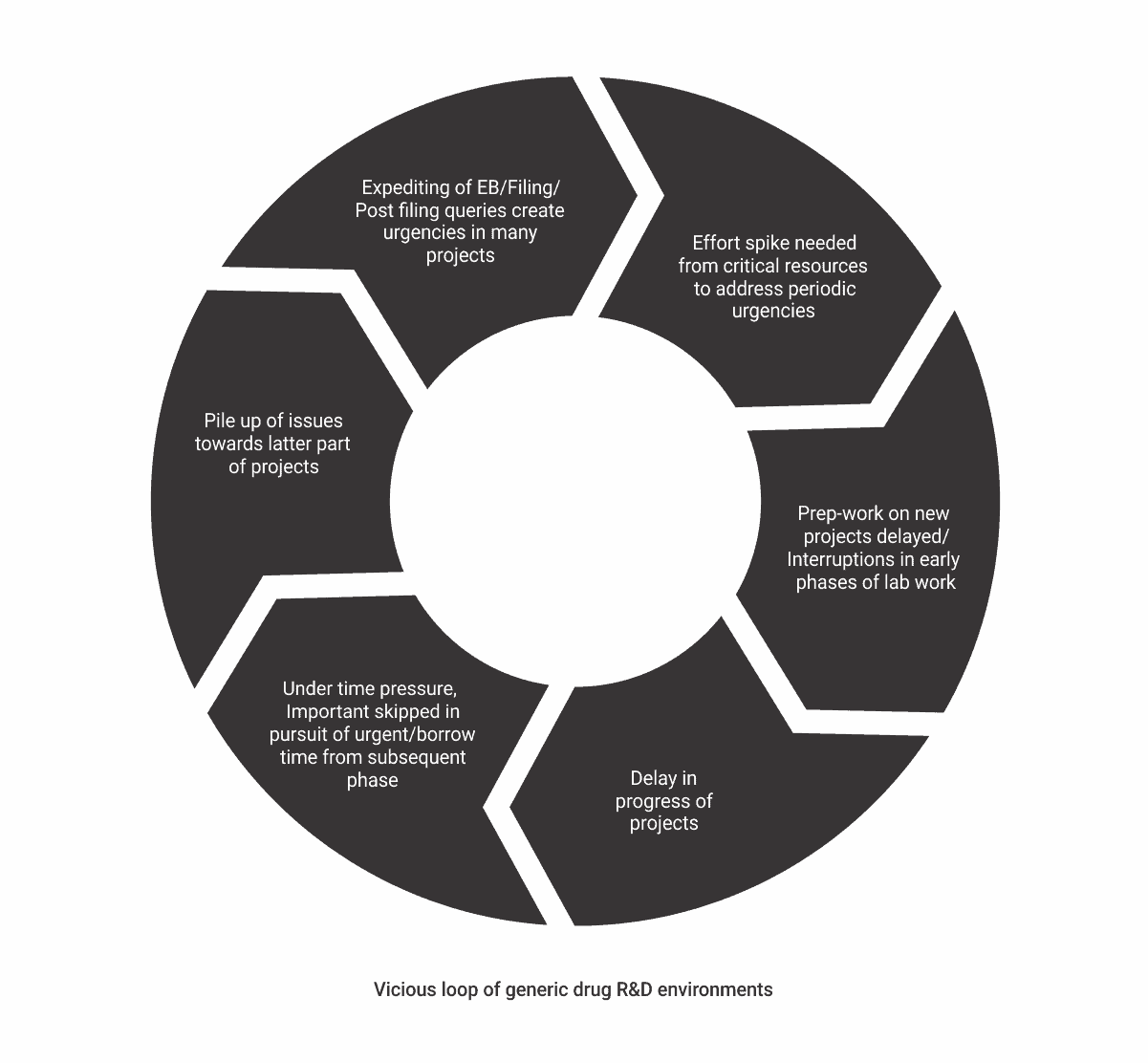
The conundrum for R&D
Since there are only so many tasks these expert resources can do at a time, they tend to focus on urgently needed ones. So, when the pressure is on submissions in the fourth quarter, they cannot pay enough attention to preparatory work for new drugs that have to be initiated for development. However, since there are date commitments to meet, these drugs move to the next stage of development even with incomplete preparation. The pressure of time due to deadlines and the consequent compromises are there at every stage of development. So, in the later stages, these slips and misses come back as interruptions and rework for almost all involved. By the time projects reach the EB stage, not only is development delayed, but the process is also often riddled with a number of open issues. However, since management attention tends to be high from the EB stage onwards, everyone is forced to focus on the urgent submission-related tasks in a firefighting mode. This is again at the cost of lab work. So, the vicious loop continues.
Elaborate planning – A futile solution
Project Managers believe that effective planning can break this vicious loop by:
- Making sure that workload and resource capacity are properly matched while creating the schedule: This is with the assumption that the time taken to accomplish a task can be determined accurately in foresight. However, this is not true since resources are differently skilled and the time that each would take for the same task will be different.
- Taking milestone commitments from resources: This is also futile since in an environment of shared resources, a delay in one project results in queuing delays across all the projects that share resources.
Solution
The way forward is to accept that traditional solutions will not work and adopt the following principles of Flow Management.
- Control WIP and clear priority: Instead of scheduling tasks, just limit projects being worked upon at a time by the whole team. So, a new project should be initiated only when the one being worked on at present is completed. Assign a sequential queue number to projects based on priority to ensure the right set of projects is given attention by all concerned.
- Ensure full kit: Preparatory work for a new project (material, information, etc.) should be completed before a new drug development is started. Similarly, each subsequent stage should meet a set of strict closure criteria before handing over work to the next team.
- High-frequency management: Establish a short, daily cross-functional meeting for issue resolution so that no task is stuck due to lack of assistance or timely decisions.
- Buffers for projects not tasks: In the project plan, have an aggregated project buffer time to account for uncertainties (instead of buffering each task). This can be monitored for proactive intervention.
Companies that have implemented these steps have experienced dramatic capacity release and lead time reduction. FDA submissions happen faster, and the workplace becomes happier!
- Advertisement -


