AI and real-world evidence: The next frontier in clinical research
As AI meets real-world evidence, clinical research is moving beyond controlled trials into the realm of real-life data.The result is a smarter, faster, and more equitable path to better healthcare, highlights Dr Seema Pai, President, Indian Society for Clinical Research
In the rapidly evolving world of clinical research, few developments hold as much promise—or provoke as much debate—as the convergence of artificial intelligence (AI) and real-world evidence (RWE). While AI has already transformed industries from finance to logistics, its ability to analyse, interpret, and act on vast datasets in real time is now redefining how we design, conduct, and scale clinical research. For India, a country with immense patient diversity, digital health penetration, and growing R&D infrastructure, this convergence presents a transformative opportunity.
The shift from hypothetical to real-world
Traditionally, randomised controlled trials (RCTs) have been the gold standard in establishing drug efficacy and safety. However, they are time-consuming, expensive, and often limited in population diversity. Real-world evidence—derived from sources like electronic health records (EHRs), insurance claims, wearable devices, and mobile health platforms— offers a complementary approach. When powered by AI, RWE can reveal patient behaviours, treatment outcomes, and adverse events in ways that RCTs simply cannot.
India is uniquely positioned to lead this shift. With over one billion mobile phone users and increasing adoption of digital health tools, there is a growing trove of real-world data waiting to be structured and analysed. AI models can mine this data to detect patterns, predict outcomes, and optimise interventions with speed and precision.
In their recent article in ISPOR – Parexel, one of the world’s largest clinical research organisations (CROs) has clearly outlined some key areas where one could use AI in clinical development as follows:
Clinical trials reimagined
There are numerous promising use cases where AI is already making an impact. For instance, AI can optimise trial protocol design by identifying ideal inclusion/exclusion criteria and predicting patient outcomes using historical and synthetic datasets. Platforms like TrialGPT and Criteria2Query have demonstrated up to 80 per cent time savings in patient screening by using natural language processing (NLP) to analyse EHRs and match patients to trials more accurately.
AI also enables the creation of virtual control arms—synthetic groups based on RWE that can reduce or eliminate the need for placebos in certain trials. This not only improves ethical considerations but also accelerates trial timelines. In oncology, algorithms are now predicting tumour responses and survival rates with increasing accuracy, supporting better endpoint definition and adaptive trial design.
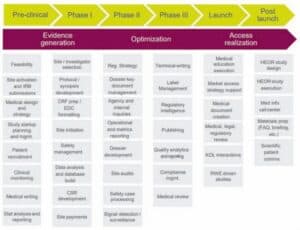
Pharmacovigilance and post-market surveillance
Real-world data is equally critical post-approval. Pharmacovigilance—a cornerstone of patient safety—is being revolutionised by AI tools that scan unstructured data like case reports, scientific literature, and social media to detect adverse events faster. AI-powered literature monitoring tools have achieved sensitivity rates as high as 97 per cent in screening for relevant safety signals.
Moreover, predictive analytics can identify at-risk populations and flag drug-drug interactions before they occur, transforming pharmacovigilance from a reactive to a proactive discipline. In India, initiatives like the Comprehensive Loss to Follow-Up and Mortality Prediction (CLAMP-TB) system for tuberculosis care show how AI can enhance patient safety and treatment adherence even at scale.
Ethical guardrails and regulatory evolution
However, innovation must be met with responsibility. AI models are only as good as the data they’re trained on. Biases in datasets can lead to exclusionary outcomes, particularly in India’s diverse population. This makes the case for explainable AI (xAI), robust anonymisation techniques, and the inclusion of human oversight in algorithmic decision-making.
On the regulatory front, India is making encouraging progress. While the Central Drugs Standard Control Organisation (CDSCO) has yet to publish formal AI guidelines, the Indian Council of Medical Research (ICMR) released ethical guidelines for AI in healthcare in 2023. These emphasise transparency, bias mitigation, and accountability—principles that must underpin every AI-powered clinical decision.
The integration of AI and RWE is not a replacement for traditional clinical research but an evolution. It opens up new paradigms—decentralised trials, real-time monitoring, population-level insights—that were previously inaccessible. It’s a future where patient safety is proactive, data is democratised, and trials reflect the true complexity of real-world conditions.
Regulatory guidelines are being developed to help shape the applications of AI across the healthcare ecosystem. EMA and USFDA have been at the forefront to highlight use of AI responsibly.
EMA states: “… the use of exceptionally great numbers of trainable parameters arranged in non-transparent model architectures introduces new risks that need to be mitigated both during model development and deployment to ensure the safety of patients and integrity of clinical study results. Also, as the overarching approach is inherently datadriven, active measures must be taken to avoid the integration of bias into AI/ML applications and promote AI trustworthiness…”
FDA states: …“There are also concerns with using algorithms that have a degree of opacity, or algorithms that may have internal operations that are not visible to users or other interested parties. This can lead to amplification of errors or preexisting biases in the data. We aim to prevent and remedy discrimination — including algorithmic discrimination, which occurs when automated systems favor one category of people over other(s) — to advance equity when using AI/ML techniques.”
Because the future of clinical research isn’t just about discovering new molecules—it’s about discovering better ways to deliver health.
The landscape is fast-evolving and collaborative efforts between AI experts and healthcare professionals and other industry stakeholders can enhance outcomes. Training /grounding these algorithms with data still requires human oversight. Adapting to the evolving regulatory landscape ensures AI system safety and efficacy; AI is positioned to accelerate clinical development and enhance patient access with continued stakeholder collaboration across the healthcare ecosystem.


