Vaccine trials essential to evaluate effectiveness against new COVID-19 variants: GlobalData
The largest proportion of the ongoing vaccine trials are now in Phase II (38.6 per cent) and Phase III (36.3 per cent), after Phase I studies dominated the earlier period of the pandemic
While governments are ramping up vaccination programmes, continued research into the effectiveness of the vaccines is imperative to prevent a worsening of the impact of COVID-19, says GlobalData.
Mohamed Abukar, Clinical Trials Analyst at GlobalData, comments, “The fast-spreading new COVID-19 variants may reduce the protective effects offered by the leading vaccines. As a result, researchers need to ascertain whether the vaccines are as effective against the new viral variants or if they require some level of modification to yield optimum immune response against the variants.”
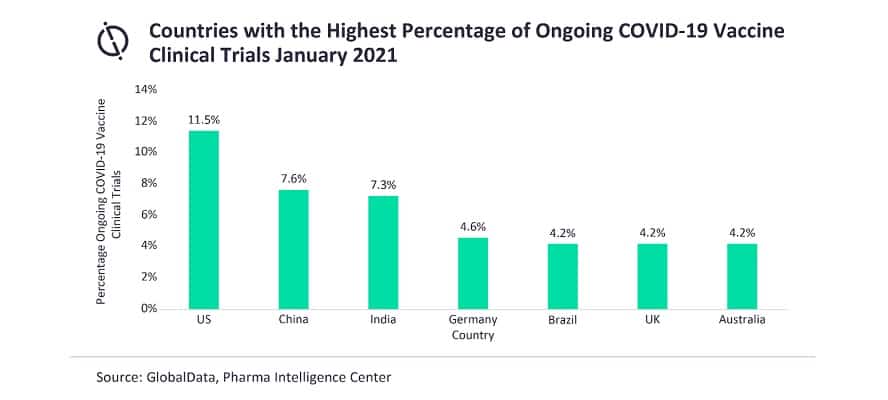
The US takes the lead in research, accounting for 11.5 per cent of the ongoing COVID-19 vaccine studies, with China following with 7.6 per cent, narrowly outnumbering India at 7.3 per cent. Germany is in fourth place with 4.6 per cent; Brazil, the UK, and Australia are tied with 4.2 per cent each.
The largest proportion of the ongoing vaccine trials are now in Phase II (38.6 per cent) and Phase III (36.3 per cent), after Phase I studies dominated the earlier period of the pandemic.
As clinical trials progress, more effectiveness results of vaccine candidates have become available. The first data released, for Moderna’s mRNA-1273 and Pfizer/BioNTech’s BNT162b2, displayed the highest efficacy levels of around 95 per cent. These vaccines model the immune response around the spike protein.
Abukar continues, “Consequently, 87.1 per cent of vaccines in the ongoing trials are now targeting the spike protein, demonstrating the high level of confidence obtained from the positive results of approved vaccine agents.”
BioNTech, Johnson & Johnson and Gamaleya are the top three trial sponsors, each with five ongoing studies. Novavax, the University of Oxford, Sinovac, Moderna, Cadila, and CureVac are each sponsoring four studies.
Abukar concludes, “A number of the listed top sponsors have already attained some level of approval for their vaccines and are continuing research to expand on knowledge of the efficacy and safety profiles of these agents. It is imperative that clinical research continues at the same pace to constantly evolve the current knowledge and adapt to new challenges such as new viral variants and ultimately to produce a universal vaccine.”
- Advertisement -


